Seawater treatment
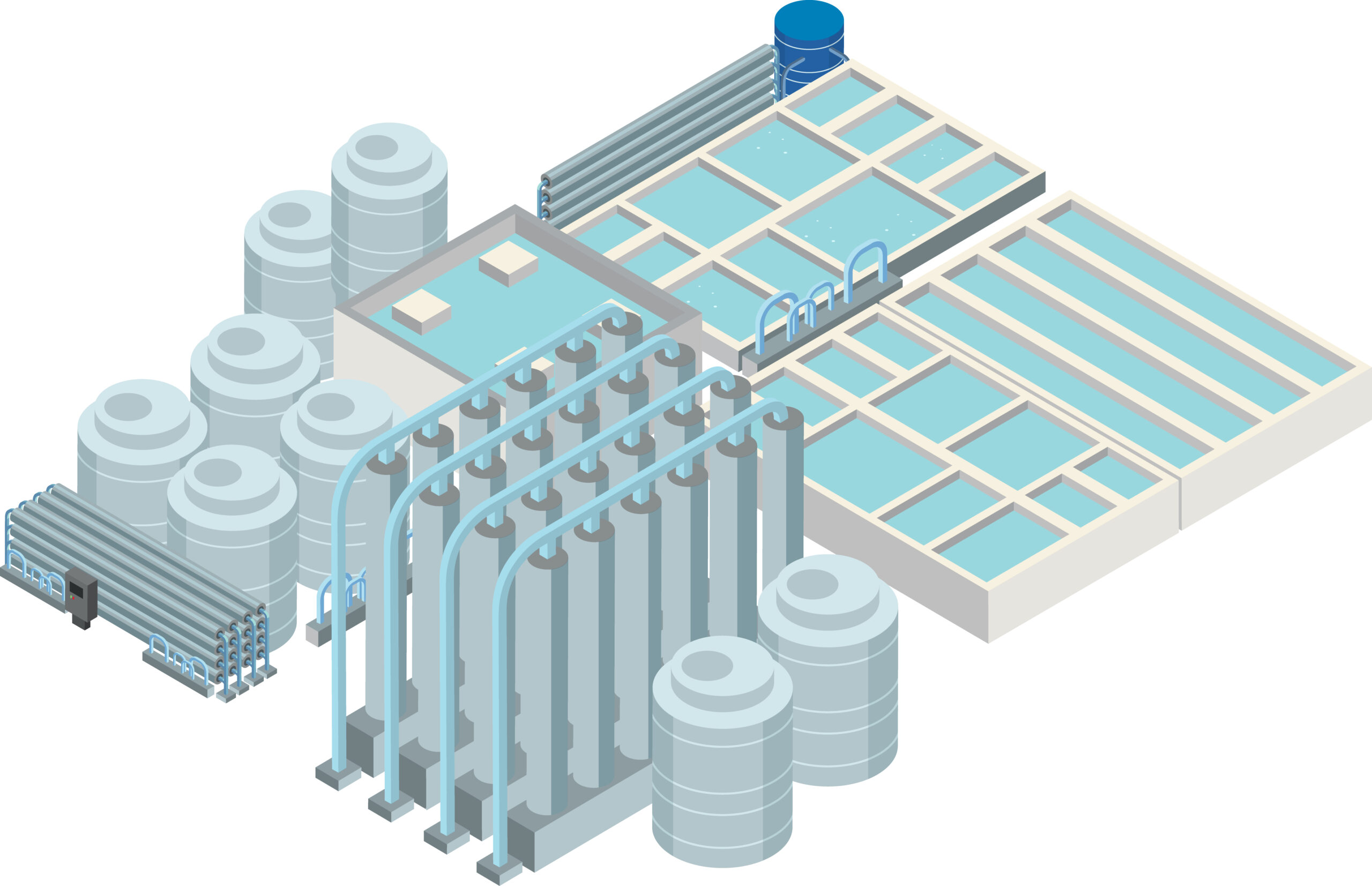
Around 96% of all global water is the salt water in the oceans. In an age of increasing scarcity of fresh water, utilisation of seawater is becoming ever more important.
With the use of suitable treatment methods such as desalination technologies, the water can be used as drinking water and process water, e.g. in the food or manufacturing industries, and in steam generation.
Desalination of seawater by reverse osmosis (RO) through membrane filtration is generally associated with a number of familiar problems.
Problem scale formation
One of these problems is scale formation, which is caused by the alkalinity (calcium hardness) of the seawater. The deposits mostly consist of insoluble calcium salts such as calcium sulphate (CaSO4) and calcium carbonate (CaCO3).
These can
- reduce the flow rate of the feed
water through pipes, - reduce the heat transfer efficiency
of heat exchangers and - reduce the productivity of
membranes and thermal processes.
Technical solutions
Currently, the three most important commercially available desalination technologies for large-scale applications are multi-stage flash distillation (MSF), multiple-effect distillation (MED) and reverse osmosis (RO).
However, these desalination technologies generally only offer a water recovery rate of 40-55%, mainly due to the problems with scale formation mentioned above.
In order to make this process as efficient as possible, precise monitoring and reduction of the calcium hardness (alkalinity) is crucial. The Testomat® Pro Ca self clean is the ideal monitoring device for this purpose.
Calcium hardness of seawater
The overall alkalinity of seawater varies between 100 and 130 mg/l of CaCO3, with an average of 116 mg/l.
Calcium hardness as CaCO3 (mg/l)
– Soft: 0-20
– Moderately soft: 20-40
– Moderately hard: 40-80
– Hard: 80-120
– Very hard: >120
